Copper
Copper is a chemical element with symbol Cu(from Latin: cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a reddish-orange color. Copper is used as a conductor of heat and electricity, as a building material, and as a constituent of various metal alloys, such as sterling silver used injewelry, cupronickel used to make marine hardware and coins, and constantan used instrain gauges and thermocouples for temperature measurement.
Copper is one of the few metals that occur in nature in directly usable metallic form (native metals) as opposed to needing extraction from an ore. This led to very early human use, from c. 8000 BC. It was the first metal to besmelted from its ore, c. 5000 BC, the first metal to be cast into a shape in a mold, c. 4000 BC and the first metal to be purposefully alloyed with another metal, tin, to createbronze, c. 3500 BC.[4]
In the Roman era, copper was principally mined on Cyprus, the origin of the name of the metal, from aes сyprium (metal of Cyprus), later corrupted to сuprum, from which the words copper (English), cuivre (French), cobre(Spanish), Koper (Dutch) and Kupfer (German) are all derived.[5] The commonly encountered compounds are copper(II) salts, which often impart blue or green colors to such minerals as azurite, malachite, and turquoise, and have been used widely and historically as pigments. Copper used in buildings, usually for roofing, oxidizes to form a green verdigris(or patina). Copper is sometimes used indecorative art, both in its elemental metal form and in compounds as pigments. Copper compounds are used as bacteriostatic agents,fungicides, and wood preservatives.
Copper is essential to all living organisms as a trace dietary mineral because it is a key constituent of the respiratory enzyme complex cytochrome c oxidase. In molluscsand crustaceans, copper is a constituent of the blood pigment hemocyanin, replaced by the iron-complexed hemoglobin in fish and other vertebrates. In humans, copper is found mainly in the liver, muscle, and bone.[6] The adult body contains between 1.4 and 2.1 mg of copper per kilogram of body weight.[7]
Characteristics
Physical

Copper just above its melting point keeps its pink luster color when enough light outshines the orange incandescencecolor
Copper, silver, and gold are in group 11 of the periodic table; these three metals have one s-orbital electron on top of a filled d-electron shell and are characterized by high ductility, and electrical and thermal conductivity. The filled d-shells in these elements contribute little to interatomic interactions, which are dominated by the s-electrons through metallic bonds. Unlike metals with incomplete d-shells, metallic bonds in copper are lacking acovalent character and are relatively weak. This observation explains the low hardnessand high ductility of single crystals of copper.[8] At the macroscopic scale, introduction of extended defects to the crystal lattice, such as grain boundaries, hinders flow of the material under applied stress, thereby increasing its hardness. For this reason, copper is usually supplied in a fine-grainedpolycrystalline form, which has greater strength than monocrystalline forms.[9]
The softness of copper partly explains its high electrical conductivity (59.6×106 S/m) and high thermal conductivity, second highest (second only to silver) among pure metals at room temperature.[10] This is because the resistivity to electron transport in metals at room temperature originates primarily from scattering of electrons on thermal vibrations of the lattice, which are relatively weak in a soft metal.[8] The maximum permissible current density of copper in open air is approximately 3.1×106 A/m2 of cross-sectional area, above which it begins to heat excessively.[11]
Copper is one of a few metallic elements with a natural color other than gray or silver.[12]Pure copper is orange-red and acquires a reddish tarnish when exposed to air. The characteristic color of copper results from the electronic transitions between the filled 3d and half-empty 4s atomic shells – the energy difference between these shells corresponds to orange light.
As with other metals, if copper is put in contact with another metal, galvanic corrosion will occur.[13]
Chemical
The East Tower of the Royal Observatory, Edinburgh. The contrast between the refurbished copper installed in 2010 and the green color of the original 1894 copper is clearly seen.
Copper does not react with water, but it does slowly react with atmospheric oxygen to form a layer of brown-black copper oxide which, unlike the rust that forms on iron in moist air, protects the underlying metal from further corrosion (passivation). A green layer ofverdigris (copper carbonate) can often be seen on old copper structures, such as the roofing of many older buildings[14] and theStatue of Liberty.[15] Copper tarnishes when exposed to some sulfur compounds, with which it reacts to form various copper sulfides.[16]
Isotopes
There are 29 isotopes of copper. 63Cu and65Cu are stable, with 63Cu comprising approximately 69% of naturally occurring copper; both have a spin of 3⁄2.[17] The other isotopes are radioactive, with the most stable being 67Cu with a half-life of 61.83 hours.[17]Seven metastable isotopes have been characterized; 68mCu is the longest-lived with a half-life of 3.8 minutes. Isotopes with amass number above 64 decay by β−, whereas those with a mass number below 64 decay byβ+. 64Cu, which has a half-life of 12.7 hours, decays both ways.[18]
62Cu and 64Cu have significant applications.62Cu is used in 62Cu-PTSM as a radioactive tracer for positron emission tomography.[19]
Occurrence
Copper is produced in massive stars[20] and is present in the Earth's crust in a proportion of about 50 parts per million (ppm).[21] In nature, copper occurs in a variety of minerals, including native copper, copper sulfides such as chalcopyrite, bornite, digenite, covellite, and chalcocite, copper sulfosalts such astetrahedite-tennantite, and enargite, copper carbonates such as azurite and malachite, and as copper(I) or copper(II) oxides such ascuprite and tenorite, respectively.[10] The largest mass of elemental copper discovered weighed 420 tonnes and was found in 1857 on the Keweenaw Peninsula in Michigan, US.[21] Native copper is a polycrystal, with the largest single crystal ever described measuring 4.4×3.2×3.2 cm.[22]
Production
Most copper is mined or extracted as copper sulfides from large open pit mines in porphyry copper deposits that contain 0.4 to 1.0% copper. Sites include Chuquicamata, in Chile,Bingham Canyon Mine, in Utah, United States, and El Chino Mine, in New Mexico, United States. According to the British Geological Survey, in 2005, Chile was the top producer of copper with at least one-third of the world share followed by the United States, Indonesia and Peru.[10] Copper can also be recovered through the in-situ leach process. Several sites in the state of Arizona are considered prime candidates for this method.[23] The amount of copper in use is increasing and the quantity available is barely sufficient to allow all countries to reach developed world levels of usage.[24]
Reserves
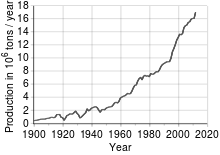
Copper has been in use at least 10,000 years, but more than 95% of all copper ever mined and smelted has been extracted since 1900,[25] and more than half was extracted the last 24 years. As with many natural resources, the total amount of copper on Earth is vast, with around 1014 tons in the top kilometer of Earth's crust, which is about 5 million years' worth at the current rate of extraction. However, only a tiny fraction of these reserves is economically viable with present-day prices and technologies. Estimates of copper reserves available for mining vary from 25 to 60 years, depending on core assumptions such as the growth rate.[26] Recycling is a major source of copper in the modern world.[25] Because of these and other factors, the future of copper production and supply is the subject of much debate, including the concept of peak copper, analogous to peak oil.
The price of copper has historically been unstable,[27] and its price increased from the 60-year low of US$0.60/lb (US$1.32/kg) in June 1999 to $3.75 per pound ($8.27/kg) in May 2006. It dropped to $2.40/lb ($5.29/kg) in February 2007, then rebounded to $3.50/lb ($7.71/kg) in April 2007.[28][better source needed] In February 2009, weakening global demand and a steep fall in commodity prices since the previous year's highs left copper prices at $1.51/lb ($3.32/kg).[29]
Methods
The concentration of copper in ores averages only 0.6%, and most commercial ores are sulfides, especially chalcopyrite (CuFeS2), bornite (Cu5FeS4) and, to a lesser extent, covellite (CuS) and chalcocite (Cu2S).[30]These minerals are concentrated fromcrushed ores to the level of 10–15% copper by froth flotation or bioleaching.[31] Heating this material with silica in flash smeltingremoves much of the iron as slag. The process exploits the greater ease of converting iron sulfides into oxides, which in turn react with the silica to form the silicateslag that floats on top of the heated mass. The resulting copper matte, consisting of Cu2S, is roasted to convert all sulfides into oxides:[30]
- 2 Cu2S + 3 O2 → 2 Cu2O + 2 SO2
The cuprous oxide is converted to blistercopper upon heating:
- 2 Cu2O → 4 Cu + O2
The Sudbury matte process converted only half the sulfide to oxide and then used this oxide to remove the rest of the sulfur as oxide. It was then electrolytically refined and the anode mud exploited for the platinum and gold it contained. This step exploits the relatively easy reduction of copper oxides to copper metal. Natural gas is blown across the blister to remove most of the remaining oxygen and electrorefining is performed on the resulting material to produce pure copper:[32]
- Cu2+ + 2 e− → Cu
Flowchart of copper refining (Anode casting plant of Uralelektromed)
- Blister copper
- Smelting
- Reverberatory furnace
- Slag removal
- Copper casting of anodes
- Casting wheel
- Anodes removal machine
- Anodes take-off
- Rail cars
- Transportation to the tank house
Recycling
Like aluminium,[33] copper is recyclable without any loss of quality, both from raw state and from manufactured products.[34] In volume, copper is the third most recycled metal after iron and aluminium.[35] An estimated 80% of all copper ever mined is still in use today.[36] According to the International Resource Panel's Metal Stocks in Society report, the global per capita stock of copper in use in society is 35–55 kg. Much of this is in more-developed countries (140–300 kg per capita) rather than less-developed countries (30–40 kg per capita).
The process of recycling copper is roughly the same as is used to extract copper but requires fewer steps. High-purity scrap copper is melted in a furnace and then reduced and cast into billets and ingots; lower-purity scrap is refined by electroplating in a bath ofsulfuric acid.[37]
Alloys
Numerous copper alloys have been formulated, many with important uses. Brassis an alloy of copper and zinc. Bronze usually refers to copper-tin alloys, but can refer to any alloy of copper such as aluminium bronze. Copper is one of the most important constituents of silver and carat gold and carat solders used in the jewelry industry, modifying the color, hardness and melting point of the resulting alloys.[38] Some lead-free soldersconsist of tin alloyed with a small proportion of copper and other metals.[39]
The alloy of copper and nickel, calledcupronickel, is used in low-denomination coins, often for the outer cladding. The US five-cent coin (currently called a nickel) consists of 75% copper and 25% nickel in homogeneous composition. The alloy of 90% copper and 10% nickel, remarkable for its resistance to corrosion, is used for various objects exposed to seawater, though it is vulnerable to the sulfides sometimes found in polluted harbors and estuaries.[40] Alloys of copper with aluminium (about 7%) have a golden color and are used in decorations.[21]Shakudō is a Japanese decorative alloy of copper containing a low percentage of gold, typically 4–10%, that can be patinated to a dark blue or black color.[41]
Compounds

A sample of copper(I) oxide.
Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively.[42]
Binary compounds
As with other elements, the simplest compounds of copper are binary compounds, i.e. those containing only two elements, the principal examples being oxides, sulfides, andhalides. Both cuprous and cupric oxides are known. Among the numerous copper sulfides, important examples include copper(I) sulfideand copper(II) sulfide.
Cuprous halides (with chlorine, bromine, andiodine) are known, as are cupric halides withfluorine, chlorine, and bromine. Attempts to prepare copper(II) iodide yield only cuprous iodide and iodine.[42]
- 2 Cu2+ + 4 I− → 2 CuI + I2
Coordination chemistry
Copper(II) gives a deep blue coloration in the presence of ammonia ligands. The one used here is tetramminecopper(II) sulfate.
Copper forms coordination complexes withligands. In aqueous solution, copper(II) exists as [Cu(H2O)6]2+. This complex exhibits the fastest water exchange rate (speed of water ligands attaching and detaching) for any transition metal aquo complex. Adding aqueous sodium hydroxide causes the precipitation of light blue solid copper(II) hydroxide. A simplified equation is:
- Cu2+ + 2 OH− → Cu(OH)2
Aqueous ammonia results in the same precipitate. Upon adding excess ammonia, the precipitate dissolves, formingtetraamminecopper(II):
- Cu(H2O)4(OH)2 + 4 NH3 → [Cu(H2O)2(NH3)4]2+ + 2 H2O + 2 OH−
Many other oxyanions form complexes; these include copper(II) acetate, copper(II) nitrate, and copper(II) carbonate. Copper(II) sulfateforms a blue crystalline pentahydrate, the most familiar copper compound in the laboratory. It is used in a fungicide called theBordeaux mixture.[43]
Ball-and-stick model of the complex [Cu(NH3)4(H2O)2]2+, illustrating the octahedral coordination geometry common for copper(II).
Polyols, compounds containing more than one alcohol functional group, generally interact with cupric salts. For example, copper salts are used to test for reducing sugars. Specifically, using Benedict's reagent andFehling's solution the presence of the sugar is signaled by a color change from blue Cu(II) to reddish copper(I) oxide.[44] Schweizer's reagent and related complexes withethylenediamine and other amines dissolvecellulose.[45] Amino acids form very stablechelate complexes with copper(II). Many wet-chemical tests for copper ions exist, one involving potassium ferrocyanide, which gives a brown precipitate with copper(II) salts.
Organocopper chemistry
Compounds that contain a carbon-copper bond are known as organocopper compounds. They are very reactive towards oxygen to form copper(I) oxide and havemany uses in chemistry. They are synthesized by treating copper(I) compounds withGrignard reagents, terminal alkynes ororganolithium reagents;[46] in particular, the last reaction described produces a Gilman reagent. These can undergo substitution withalkyl halides to form coupling products; as such, they are important in the field of organic synthesis. Copper(I) acetylide is highly shock-sensitive but is an intermediate in reactions such as the Cadiot-Chodkiewicz coupling[47]and the Sonogashira coupling.[48] Conjugate addition to enones[49] and carbocupration of alkynes[50] can also be achieved with organocopper compounds. Copper(I) forms a variety of weak complexes with alkenes andcarbon monoxide, especially in the presence of amine ligands.[51]
Copper(III) and copper(IV)
Copper(III) is most often found in oxides. A simple example is potassium cuprate, KCuO2, a blue-black solid.[52] The most extensively studied copper(III) compounds are thecuprate superconductors. Yttrium barium copper oxide (YBa2Cu3O7) consists of both Cu(II) and Cu(III) centres. Like oxide, fluorideis a highly basic anion[53] and is known to stabilize metal ions in high oxidation states. Both copper(III) and even copper(IV) fluorides are known, K3CuF6 and Cs2CuF6, respectively.[42]
Some copper proteins form oxo complexes, which also feature copper(III).[54] Withtetrapeptides, purple-colored copper(III) complexes are stabilized by the deprotonatedamide ligands.[55]
Complexes of copper(III) are also found as intermediates in reactions of organocopper compounds.[56] For example, in theKharasch–Sosnovsky reaction.






Comments
Post a Comment