Steel
Steel is an alloy of iron and carbon and other elements. Because of its high tensile strengthand low cost, it is a major component used inbuildings, infrastructure, tools, ships,automobiles, machines, appliances, andweapons.
Iron is the base metal of steel. Iron is able to take on two crystalline forms (allotropic forms), body centered cubic (BCC) and face centered cubic (FCC), depending on its temperature. In the body-centred cubic arrangement, there is an iron atom in the centre of each cube, and in the face-centred cubic, there is one at the center of each of the six faces of the cube. It is the interaction of the allotropes of iron with the alloying elements, primarily carbon, that gives steel and cast iron their range of unique properties.
In pure iron, the crystal structure has relatively little resistance to the iron atoms slipping past one another, and so pure iron is quiteductile, or soft and easily formed. In steel, small amounts of carbon, other elements, and inclusions within the iron act as hardening agents that prevent the movement ofdislocations that are common in the crystal lattices of iron atoms.
The carbon in typical steel alloys may contribute up to 2.14% of its weight. Varying the amount of carbon and many other alloying elements, as well as controlling their chemical and physical makeup in the final steel (either as solute elements, or as precipitated phases), slows the movement of those dislocations that make pure iron ductile, and thus controls and enhances its qualities. These qualities include such things as thehardness, quenching behavior, need forannealing, tempering behavior, yield strength, and tensile strength of the resulting steel. The increase in steel's strength compared to pure iron is possible only by reducing iron'sductility.
Steel was produced in bloomery furnaces for thousands of years, but its large-scale, industrial use began only after more efficient production methods were devised in the 17th century, with the production of blister steeland then crucible steel. With the invention of the Bessemer process in the mid-19th century, a new era of mass-produced steel began. This was followed by the Siemens-Martin process and then the Gilchrist-Thomas process that refined the quality of steel. With their introductions, mild steel replacedwrought iron.
Further refinements in the process, such asbasic oxygen steelmaking (BOS), largely replaced earlier methods by further lowering the cost of production and increasing the quality of the final product. Today, steel is one of the most common man-made materials in the world, with more than 1.6 billion tons produced annually. Modern steel is generally identified by various grades defined by assorted standards organizations.
The noun steel originates from the Proto-Germanic adjective stahliją or stakhlijan(made of steel), which is related to stahlaz orstahliją (standing firm).[1]
The carbon content of steel is between 0.002% and 2.14% by weight for plainiron–carbon alloys.[2] These values vary depending on alloying elements such asmanganese, chromium, nickel, iron, tungsten, carbon and so on. Basically, steel is an iron-carbon alloy that does not undergo eutectic reaction. In contrast, cast iron does undergo eutectic reaction. Too little carbon content leaves (pure) iron quite soft, ductile, and weak. Carbon contents higher than those of steel make a brittle alloy commonly called pig iron. While iron alloyed with carbon is called carbon steel, alloy steel is steel to which other alloying elements have been intentionally added to modify the characteristics of steel. Common alloying elements include: manganese, nickel, chromium, molybdenum,boron, titanium, vanadium, tungsten, cobalt, and niobium.[3] Additional elements are also important in steel: phosphorus, sulfur, silicon, and traces of oxygen, nitrogen, and copper, that are most frequently considered undesirable.
Plain carbon-iron alloys with a higher than 2.1% carbon content are known as cast iron. With modern steelmaking techniques such as powder metal forming, it is possible to make very high-carbon (and other alloy material) steels, but such are not common. Cast iron is not malleable even when hot, but it can be formed by casting as it has a lower melting point than steel and good castabilityproperties.[3] Certain compositions of cast iron, while retaining the economies of melting and casting, can be heat treated after casting to make malleable iron or ductile iron objects. Steel is distinguishable from wrought iron(now largely obsolete), which may contain a small amount of carbon but large amounts ofslag.
Material properties
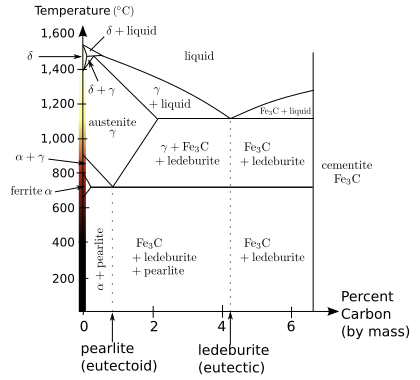
Iron-carbon phase diagram, showing the conditions necessary to form different phases
Iron is commonly found in the Earth's crust in the form of an ore, usually an iron oxide, such as magnetite or hematite. Iron is extracted from iron ore by removing the oxygen through its combination with a preferred chemical partner such as carbon which is then lost to the atmosphere as carbon dioxide. This process, known as smelting, was first applied to metals with lower melting points, such astin, which melts at about 250 °C (482 °F), andcopper, which melts at about 1,100 °C (2,010 °F), and the combination, bronze, which has a melting point lower than 1,083 °C (1,981 °F). In comparison, cast iron melts at about 1,375 °C (2,507 °F).[4] Small quantities of iron were smelted in ancient times, in the solid state, by heating the ore in a charcoalfire and then welding the clumps together with a hammer and in the process squeezing out the impurities. With care, the carbon content could be controlled by moving it around in the fire. Unlike copper and tin, liquid or solid iron dissolves carbon quite readily.
All of these temperatures could be reached with ancient methods used since the Bronze Age. Since the oxidation rate of iron increases rapidly beyond 800 °C (1,470 °F), it is important that smelting take place in a low-oxygen environment. Smelting, using carbon to reduce iron oxides, results in an alloy (pig iron) that retains too much carbon to be called steel.[4] The excess carbon and other impurities are removed in a subsequent step.
Other materials are often added to the iron/carbon mixture to produce steel with desired properties. Nickel and manganese in steel add to its tensile strength and make theaustenite form of the iron-carbon solution more stable, chromium increases hardness and melting temperature, and vanadium also increases hardness while making it less prone to metal fatigue.[5]
To inhibit corrosion, at least 11% chromium is added to steel so that a hard oxide forms on the metal surface; this is known as stainless steel. Tungsten slows the formation ofcementite, keeping carbon in the iron matrix and allowing martensite to preferentially form at slower quench rates, resulting in high speed steel. On the other hand, sulfur,nitrogen, and phosphorus are considered contaminants that make steel more brittle and are removed from the steel melt during processing.[5]
The density of steel varies based on the alloying constituents but usually ranges between 7,750 and 8,050 kg/m3 (484 and 503 lb/cu ft), or 7.75 and 8.05 g/cm3 (4.48 and 4.65 oz/cu in).[6]
Even in a narrow range of concentrations of mixtures of carbon and iron that make a steel, a number of different metallurgical structures, with very different properties can form. Understanding such properties is essential to making quality steel. At room temperature, the most stable form of pure iron is the body-centered cubic (BCC) structure called alpha iron or α-iron. It is a fairly soft metal that can dissolve only a small concentration of carbon, no more than 0.005% at 0 °C (32 °F) and 0.021 wt% at 723 °C (1,333 °F). The inclusion of carbon in alpha iron is called ferrite. At 910 °C pure iron transforms into a face-centered cubic (FCC) structure, called gamma iron or γ-iron. The inclusion of carbon in gamma iron is called austenite. The more open FCC structure of austenite can dissolve considerably more carbon, as much as 2.1%[7](38 times that of ferrite) carbon at 1,148 °C (2,098 °F), which reflects the upper carbon content of steel, beyond which is cast iron.[8]When carbon moves out of solution with iron it forms a very hard, but brittle material called cementite (Fe3C).
When steels with exactly 0.8% carbon (known as a eutectoid steel), are cooled, theaustenitic phase (FCC) of the mixture attempts to revert to the ferrite phase (BCC). The carbon no longer fits within the FCC austenite structure, resulting in an excess of carbon. One way for carbon to leave the austenite is for it to precipitate out of solution as cementite, leaving behind a surrounding phase of BCC iron called ferrite with a small percentage of carbon in solution. The two, ferrite and cementite, precipitate simultaneously producing a layered structure called pearlite, named for its resemblance tomother of pearl. In a hypereutectoid composition (greater than 0.8% carbon), the carbon will first precipitate out as large inclusions of cementite at the austenite grain boundaries until the percenage of carbon in the grains has decreased to the eutectoid composition (0.8% carbon), at which point the pearlite structure forms. For steels that have less than 0.8% carbon (hypoeutectoid), ferrite will first form within the grains until the remaining composition rises to 0.8% of carbon, at which point the pearlite structure will form. No large inclusions of cementite will form at the boundaries in hypoeuctoid steel.[9]The above assumes that the cooling process is very slow, allowing enough time for the carbon to migrate.
As the rate of cooling is increased the carbon will have less time to migrate to form carbide at the grain boundaries but will have increasingly large amounts of pearlite of a finer and finer structure within the grains; hence the carbide is more widely dispersed and acts to prevent slip of defects within those grains, resulting in hardening of the steel. At the very high cooling rates produced by quenching, the carbon has no time to migrate but is locked within the face-centered austenite and forms martensite. Martensite is a highly strained and stressed, supersaturated form of carbon and iron and is exceedingly hard but brittle. Depending on the carbon content, the martensitic phase takes different forms. Below 0.2% carbon, it takes on a ferrite BCC crystal form, but at higher carbon content it takes a body-centered tetragonal(BCT) structure. There is no thermal activation energy for the transformation from austenite to martensite.[clarification needed] Moreover, there is no compositional change so the atoms generally retain their same neighbors.[10]
Martensite has a lower density (it expands during the cooling) than does austenite, so that the transformation between them results in a change of volume. In this case, expansion occurs. Internal stresses from this expansion generally take the form of compression on the crystals of martensite and tension on the remaining ferrite, with a fair amount of shearon both constituents. If quenching is done improperly, the internal stresses can cause a part to shatter as it cools. At the very least, they cause internal work hardening and other microscopic imperfections. It is common for quench cracks to form when steel is water quenched, although they may not always be visible.[11]
Heat treatment
There are many types of heat treatingprocesses available to steel. The most common are annealing, quenching, andtempering. Heat treatment is effective on compositions above the eutectoid composition (hypereutectoid) of 0.8% carbon. Hypoeutectoid steel does not benefit from heat treatment.
Annealing is the process of heating the steel to a sufficiently high temperature to relieve local internal stresses. It does not create a general softening of the product but only locally relieves strains and stresses locked up within the material. Annealing goes through three phases: recovery, recrystallization, andgrain growth. The temperature required to anneal a particular steel depends on the type of annealing to be achieved and the alloying constituents.[12]
Quenching involves heating the steel to create the austenite phase then quenching it in water or oil. This rapid cooling results in a hard but brittle martensitic structure.[10] The steel is then tempered, which is just a specialized type of annealing, to reduce brittleness. In this application the annealing (tempering) process transforms some of the martensite into cementite, or spheroidite and hence it reduces the internal stresses and defects. The result is a more ductile and fracture-resistant steel.[13]
Steel production

Iron ore pellets for the production of steel
When iron is smelted from its ore, it contains more carbon than is desirable. To become steel, it must be reprocessed to reduce the carbon to the correct amount, at which point other elements can be added. In the past, steel facilities would cast the raw steel product into ingots which would be stored until use in further refinement processes that resulted in the finished product. In modern facilities, the initial product is close to the final composition and is continuously castinto long slabs, cut and shaped into bars and extrusions and heat treated to produce a final product. Today only a small fraction is castinto ingots. Approximately 96% of steel is continuously cast, while only 4% is produced as ingots.[14]
The ingots are then heated in a soaking pit and hot rolled into slabs, billets, or blooms. Slabs are hot or cold rolled into sheet metal or plates. Billets are hot or cold rolled into bars, rods, and wire. Blooms are hot or cold rolled into structural steel, such as I-beams andrails. In modern steel mills these processes often occur in one assembly line, with ore coming in and finished steel products coming out.[15] Sometimes after a steel's final rolling it is heat treated for strength, however this is relatively rare.[16]
History of steelmaking
Bloomery smelting during the Middle Ages
Ancient steel
The earliest known production of steel is seen in pieces of ironware excavated from anarchaeological site in Anatolia (Kaman-Kalehoyuk) and are nearly 4,000 years old, dating from 1800 BC.[19][20] Horace identifies steel weapons such as the falcata in theIberian Peninsula, while Noric steel was used by the Roman military.[21]
The reputation of Seric iron of South India (wootz steel) grew considerably in the rest of the world.[18] Metal production sites in Sri Lanka employed wind furnaces driven by the monsoon winds, capable of producing high-carbon steel. Large-scale Wootz steelproduction in Tamilakam using crucibles and carbon sources such as the plant Avāramoccurred by the sixth century BC, the pioneering precursor to modern steel production and metallurgy.[17][18]
The Chinese of the Warring States period(403–221 BC) had quench-hardened steel,[22]while Chinese of the Han dynasty (202 BC – 220 AD) created steel by melting together wrought iron with cast iron, gaining an ultimate product of a carbon-intermediate steel by the 1st century AD.[23][24]
Wootz steel and Damascus steel
Evidence of the earliest production of high carbon steel in the Indian Subcontinent are found in Kodumanal in Tamil Nadu area,Golconda in Andhra Pradesh area andKarnataka, and in Samanalawewa areas of Sri Lanka.[25] This came to be known as Wootz steel, produced in South India by about sixth century BC and exported globally.[26][27] The steel technology existed prior to 326 BC in the region as they are mentioned in literature ofSangam Tamil, Arabic and Latin as the finest steel in the world exported to the Romans, Egyptian, Chinese and Arab worlds at that time – what they called Seric Iron.[28] A 200 BC Tamil trade guild in Tissamaharama, in the South East of Sri Lanka, brought with them some of the oldest iron and steel artifacts and production processes to the island from theclassical period.[29][30][31] The Chinese and locals in Anuradhapura, Sri Lanka had also adopted the production methods of creating Wootz steel from the Chera Dynasty Tamils of South India by the 5th century AD.[32][33] In Sri Lanka, this early steel-making method employed a unique wind furnace, driven by the monsoon winds, capable of producing high-carbon steel.[34][35] Since the technology was acquired from the Tamilians from South India,[citation needed] the origin of steel technology in India can be conservatively estimated at 400–500 BC.[26][35]
The manufacture of what came to be called Wootz, or Damascus steel, famous for its durability and ability to hold an edge, may have been taken by the Arabs from Persia, who took it from India. It was originally created from a number of different materials including various trace elements, apparently ultimately from the writings of Zosimos of Panopolis. In 327 BCE, Alexander the Greatwas rewarded by the defeated King Porus, not with gold or silver but with 30 pounds of steel.[36] Recent studies have suggested thatcarbon nanotubes were included in its structure, which might explain some of its legendary qualities, though given the technology of that time, such qualities were produced by chance rather than by design.[37]Natural wind was used where the soil containing iron was heated by the use of wood. The ancient Sinhalese managed to extract a ton of steel for every 2 tons of soil,[34] a remarkable feat at the time. One such furnace was found in Samanalawewa and archaeologists were able to produce steel as the ancients did.[34][38]
Crucible steel, formed by slowly heating and cooling pure iron and carbon (typically in the form of charcoal) in a crucible, was produced in Merv by the 9th to 10th century AD.[27] In the 11th century, there is evidence of the production of steel in Song China using two techniques: a "berganesque" method that produced inferior, inhomogeneous, steel, and a precursor to the modern Bessemer processthat used partial decarbonization via repeated forging under a cold blast.[39]
Modern steelmaking
A Bessemer converter inSheffield, England
Since the 17th century, the first step in European steel production has been the smelting of iron ore into pig iron in a blast furnace.[40] Originally employing charcoal, modern methods use coke, which has proven more economical.[41][42][43]
Processes starting from bar iron
In these processes pig iron was refined (fined) in a finery forge to produce bar iron, which was then used in steel-making.[40]
The production of steel by the cementation process was described in a treatise published in Prague in 1574 and was in use inNuremberg from 1601. A similar process forcase hardening armour and files was described in a book published in Naples in 1589. The process was introduced to England in about 1614 and used to produce such steel by Sir Basil Brooke at Coalbrookdale during the 1610s.[44]
The raw material for this process were bars of iron. During the 17th century it was realized that the best steel came from oregrounds ironof a region north of Stockholm, Sweden. This was still the usual raw material source in the 19th century, almost as long as the process was used.[45][46]
Crucible steel is steel that has been melted in a crucible rather than having been forged, with the result that it is more homogeneous. Most previous furnaces could not reach high enough temperatures to melt the steel. The early modern crucible steel industry resulted from the invention of Benjamin Huntsman in the 1740s. Blister steel (made as above) was melted in a crucible or in a furnace, and cast (usually) into ingots.[46][47]
Processes starting from pig iron
A Siemens-Martin steel oven from theBrandenburg Museum of Industry.
The modern era in steelmaking began with the introduction of Henry Bessemer's Bessemer process in 1855, the raw material for which was pig iron.[48] His method let him produce steel in large quantities cheaply, thus mild steel came to be used for most purposes for which wrought iron was formerly used.[49] The Gilchrist-Thomas process (or basic Bessemer process) was an improvement to the Bessemer process, made by lining the converter with a basic material to remove phosphorus.
Another 19th-century steelmaking process was the Siemens-Martin process, which complemented the Bessemer process.[46] It consisted of co-melting bar iron (or steel scrap) with pig iron.
These methods of steel production were rendered obsolete by the Linz-Donawitz process of basic oxygen steelmaking (BOS), developed in the 1950s, and other oxygen steel making methods. Basic oxygen steelmaking is superior to previous steelmaking methods because the oxygen pumped into the furnace limited impurities, primarily nitrogen, that previously had entered from the air used.[50] Today, electric arc furnaces (EAF) are a common method of reprocessing scrap metal to create new steel. They can also be used for converting pig iron to steel, but they use a lot of electrical energy (about 440 kWh per metric ton), and are thus generally only economical when there is a plentiful supply of cheap electricity.[51]


Comments
Post a Comment