Metabolism
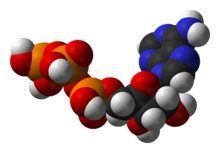
Structure of adenosine triphosphate(ATP), a central intermediate in energy metabolism
Metabolism (from Greek: μεταβολή metabolē, "change") is the set of life-sustaining chemical transformations within the cells of organisms. The three main purposes of metabolism are the conversion of food/fuel to energy to run cellular processes, the conversion of food/fuel to building blocks for proteins,lipids, nucleic acids, and some carbohydrates, and the elimination of nitrogenous wastes. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to the sum of all chemical reactions that occur in living organisms, including digestionand the transport of substances into and between different cells, in which case the set of reactions within the cells is calledintermediary metabolism or intermediate metabolism.
Metabolism is usually divided into two categories: catabolism, the breaking down of organic matter for example, the breaking down of glucose to pyruvate, by cellular respiration, and anabolism, the building up of components of cells such as proteins andnucleic acids. Usually, breaking down releasesenergy and building up consumes energy.
The chemical reactions of metabolism are organized into metabolic pathways, in which one chemical is transformed through a series of steps into another chemical, by a sequence of enzymes. Enzymes are crucial to metabolism because they allow organisms to drive desirable reactions that require energythat will not occur by themselves, by couplingthem to spontaneous reactions that release energy. Enzymes act as catalysts that allow the reactions to proceed more rapidly. Enzymes also allow the regulation of metabolic pathways in response to changes in the cell's environment or to signals from other cells.
The metabolic system of a particular organism determines which substances it will find nutritious and which poisonous. For example, some prokaryotes use hydrogen sulfide as a nutrient, yet this gas is poisonous to animals.[1] The speed of metabolism, themetabolic rate, influences how much food an organism will require, and also affects how it is able to obtain that food.
A striking feature of metabolism is the similarity of the basic metabolic pathways and components between even vastly different species.[2] For example, the set ofcarboxylic acids that are best known as the intermediates in the citric acid cycle are present in all known organisms, being found in species as diverse as the unicellularbacterium Escherichia coli and hugemulticellular organisms like elephants.[3]These striking similarities in metabolic pathways are likely due to their early appearance in evolutionary history, and their retention because of their efficacy.[4][5]
Key biochemicals
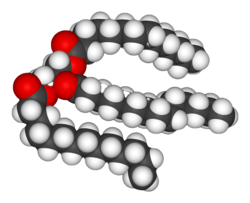
Structure of a triacylglycerol lipid
Most of the structures that make up animals, plants and microbes are made from three basic classes of molecule: amino acids,carbohydrates and lipids (often called fats). As these molecules are vital for life, metabolic reactions either focus on making these molecules during the construction of cells and tissues, or by breaking them down and using them as a source of energy, by their digestion. These biochemicals can be joined together to make polymers such as DNA andproteins, essential macromolecules of life.
Amino acids and proteins
Proteins are made of amino acids arranged in a linear chain joined together by peptide bonds. Many proteins are enzymes thatcatalyze the chemical reactions in metabolism. Other proteins have structural or mechanical functions, such as those that form the cytoskeleton, a system ofscaffolding that maintains the cell shape.[6]Proteins are also important in cell signaling,immune responses, cell adhesion, active transport across membranes, and the cell cycle.[7] Amino acids also contribute to cellular energy metabolism by providing a carbon source for entry into the citric acid cycle (tricarboxylic acid cycle),[8] especially when a primary source of energy, such asglucose, is scarce, or when cells undergo metabolic stress.[9]
Lipids
Lipids are the most diverse group of biochemicals. Their main structural uses are as part of biological membranes both internal and external, such as the cell membrane, or as a source of energy.[7] Lipids are usually defined as hydrophobic or amphipathicbiological molecules but will dissolve inorganic solvents such as benzene orchloroform.[10] The fats are a large group of compounds that contain fatty acids andglycerol; a glycerol molecule attached to three fatty acid esters is called a triacylglyceride.[11]Several variations on this basic structure exist, including alternate backbones such assphingosine in the sphingolipids, andhydrophilic groups such as phosphate as inphospholipids. Steroids such as cholesterolare another major class of lipids.[12]
Carbohydrates
Glucose can exist in both a straight-chain and ring form.
Carbohydrates are aldehydes or ketones, with many hydroxyl groups attached, that can exist as straight chains or rings. Carbohydrates are the most abundant biological molecules, and fill numerous roles, such as the storage and transport of energy (starch, glycogen) and structural components (cellulose in plants,chitin in animals).[7] The basic carbohydrate units are called monosaccharides and includegalactose, fructose, and most importantlyglucose. Monosaccharides can be linked together to form polysaccharides in almost limitless ways.[13]
Nucleotides
The two nucleic acids, DNA and RNA, are polymers of nucleotides. Each nucleotide is composed of a phosphate attached to aribose or deoxyribose sugar group which is attached to a nitrogenous base. Nucleic acids are critical for the storage and use of genetic information, and its interpretation through the processes of transcription and protein biosynthesis.[7] This information is protected by DNA repair mechanisms and propagated through DNA replication. Many viruses have an RNA genome, such as HIV, which usesreverse transcription to create a DNA template from its viral RNA genome.[14] RNA in ribozymes such as spliceosomes andribosomes is similar to enzymes as it can catalyze chemical reactions. Individualnucleosides are made by attaching anucleobase to a ribose sugar. These bases are heterocyclic rings containing nitrogen, classified as purines or pyrimidines. Nucleotides also act as coenzymes in metabolic-group-transfer reactions.[15]
Coenzymes
Structure of the coenzyme acetyl-CoA.The transferable acetyl group is bonded to the sulfur atom at the extreme left.
Metabolism involves a vast array of chemical reactions, but most fall under a few basic types of reactions that involve the transfer offunctional groups of atoms and their bonds within molecules.[16] This common chemistry allows cells to use a small set of metabolic intermediates to carry chemical groups between different reactions.[15] These group-transfer intermediates are called coenzymes. Each class of group-transfer reactions is carried out by a particular coenzyme, which is the substrate for a set of enzymes that produce it, and a set of enzymes that consume it. These coenzymes are therefore continuously made, consumed and then recycled.[17]
One central coenzyme is adenosine triphosphate (ATP), the universal energy currency of cells. This nucleotide is used to transfer chemical energy between different chemical reactions. There is only a small amount of ATP in cells, but as it is continuously regenerated, the human body can use about its own weight in ATP per day.[17] ATP acts as a bridge betweencatabolism and anabolism. Catabolism breaks down molecules, and anabolism puts them together. Catabolic reactions generate ATP, and anabolic reactions consume it. It also serves as a carrier of phosphate groups in phosphorylation reactions.
A vitamin is an organic compound needed in small quantities that cannot be made in cells. In human nutrition, most vitamins function as coenzymes after modification; for example, all water-soluble vitamins are phosphorylated or are coupled to nucleotides when they are used in cells.[18] Nicotinamide adenine dinucleotide (NAD+), a derivative of vitamin B3(niacin), is an important coenzyme that acts as a hydrogen acceptor. Hundreds of separate types of dehydrogenases remove electrons from their substrates and reduceNAD+ into NADH. This reduced form of the coenzyme is then a substrate for any of thereductases in the cell that need to reduce their substrates.[19] Nicotinamide adenine dinucleotide exists in two related forms in the cell, NADH and NADPH. The NAD+/NADH form is more important in catabolic reactions, while NADP+/NADPH is used in anabolic reactions.
Structure of hemoglobin. The protein subunits are in red and blue, and the iron-containing heme groups in green. From PDB: 1GZX.
Minerals and cofactors
Inorganic elements play critical roles in metabolism; some are abundant (e.g. sodiumand potassium) while others function at minute concentrations. About 99% of a mammal's mass is made up of the elementscarbon, nitrogen, calcium, sodium, chlorine,potassium, hydrogen, phosphorus, oxygenand sulfur.[20] Organic compounds (proteins, lipids and carbohydrates) contain the majority of the carbon and nitrogen; most of the oxygen and hydrogen is present as water.[20]
The abundant inorganic elements act as ionicelectrolytes. The most important ions aresodium, potassium, calcium, magnesium,chloride, phosphate and the organic ionbicarbonate. The maintenance of precise ion gradients across cell membranes maintainsosmotic pressure and pH.[21] Ions are also critical for nerve and muscle function, asaction potentials in these tissues are produced by the exchange of electrolytes between the extracellular fluid and the cell's fluid, the cytosol.[22] Electrolytes enter and leave cells through proteins in the cell membrane called ion channels. For example,muscle contraction depends upon the movement of calcium, sodium and potassium through ion channels in the cell membrane and T-tubules.[23]
Transition metals are usually present as trace elements in organisms, with zinc and ironbeing most abundant of those.[24][25] These metals are used in some proteins ascofactors and are essential for the activity of enzymes such as catalase and oxygen-carrier proteins such as hemoglobin.[26] Metal cofactors are bound tightly to specific sites in proteins; although enzyme cofactors can be modified during catalysis, they always return to their original state by the end of the reaction catalyzed. Metal micronutrients are taken up into organisms by specific transporters and bind to storage proteins such as ferritin or metallothionein when not in use.[27][28]
Catabolism
Catabolism is the set of metabolic processes that break down large molecules. These include breaking down and oxidizing food molecules. The purpose of the catabolic reactions is to provide the energy and components needed by anabolic reactions which build molecules. The exact nature of these catabolic reactions differ from organism to organism, and organisms can be classified based on their sources of energy and carbon (their primary nutritional groups), as shown in the table below. Organic molecules are used as a source of energy byorganotrophs, while lithotrophs use inorganic substrates, and phototrophs capture sunlight as chemical energy. However, all these different forms of metabolism depend onredox reactions that involve the transfer of electrons from reduced donor molecules such as organic molecules, water, ammonia,hydrogen sulfide or ferrous ions to acceptor molecules such as oxygen, nitrate orsulfate.[29] In animals, these reactions involve complex organic molecules that are broken down to simpler molecules, such as carbon dioxide and water. In photosyntheticorganisms, such as plants and cyanobacteria, these electron-transfer reactions do not release energy but are used as a way of storing energy absorbed from sunlight.[30]
The most common set of catabolic reactions in animals can be separated into three main stages. In the first stage, large organic molecules, such as proteins, polysaccharidesor lipids, are digested into their smaller components outside cells. Next, these smaller molecules are taken up by cells and converted to smaller molecules, usually acetyl coenzyme A (acetyl-CoA), which releases some energy. Finally, the acetyl group on the CoA is oxidised to water and carbon dioxide in the citric acid cycle and electron transport chain, releasing the energy that is stored by reducing the coenzyme nicotinamide adenine dinucleotide (NAD+) into NADH.
Digestion
Macromolecules such as starch, cellulose or proteins cannot be rapidly taken up by cells and must be broken into their smaller units before they can be used in cell metabolism. Several common classes of enzymes digest these polymers. These digestive enzymesinclude proteases that digest proteins into amino acids, as well as glycoside hydrolasesthat digest polysaccharides into simple sugars known as monosaccharides.
Microbes simply secrete digestive enzymes into their surroundings,[31][32] while animals only secrete these enzymes from specialized cells in their guts, including the stomach andpancreas, and salivary glands.[33] The amino acids or sugars released by these extracellular enzymes are then pumped into cells by active transport proteins.[34][35]
Energy from organic compounds
Carbohydrate catabolism is the breakdown of carbohydrates into smaller units. Carbohydrates are usually taken into cells once they have been digested intomonosaccharides.[36] Once inside, the major route of breakdown is glycolysis, where sugars such as glucose and fructose are converted into pyruvate and some ATP is generated.[37] Pyruvate is an intermediate in several metabolic pathways, but the majority is converted to acetyl-CoA through aerobic (with oxygen) glycolysis and fed into the citric acid cycle. Although some more ATP is generated in the citric acid cycle, the most important product is NADH, which is made from NAD+ as the acetyl-CoA is oxidized. This oxidation releases carbon dioxide as a waste product. In anaerobic conditions, glycolysis produces lactate, through the enzyme lactate dehydrogenase re-oxidizing NADH to NAD+ for re-use in glycolysis. An alternative route for glucose breakdown is the pentose phosphate pathway, which reduces the coenzyme NADPH and produces pentosesugars such as ribose, the sugar component of nucleic acids.
Fats are catabolised by hydrolysis to free fatty acids and glycerol. The glycerol enters glycolysis and the fatty acids are broken down by beta oxidation to release acetyl-CoA, which then is fed into the citric acid cycle. Fatty acids release more energy upon oxidation than carbohydrates because carbohydrates contain more oxygen in their structures. Steroids are also broken down by some bacteria in a process similar to beta oxidation, and this breakdown process involves the release of significant amounts of acetyl-CoA, propionyl-CoA, and pyruvate, which can all be used by the cell for energy. M. tuberculosiscan also grow on the lipid cholesterol as a sole source of carbon, and genes involved in the cholesterol use pathway(s) have been validated as important during various stages of the infection lifecycle of M. tuberculosis.[38]
Amino acids are either used to synthesize proteins and other biomolecules, or oxidized to urea and carbon dioxide as a source of energy.[39] The oxidation pathway starts with the removal of the amino group by atransaminase. The amino group is fed into theurea cycle, leaving a deaminated carbon skeleton in the form of a keto acid. Several of these keto acids are intermediates in the citric acid cycle, for example the deamination ofglutamate forms α-ketoglutarate.[40] Theglucogenic amino acids can also be converted into glucose, throughgluconeogenesis (discussed below).[41]
Energy transformations
Oxidative phosphorylation
In oxidative phosphorylation, the electrons removed from organic molecules in areas such as the protagon acid cycle are transferred to oxygen and the energy released is used to make ATP. This is done ineukaryotes by a series of proteins in the membranes of mitochondria called theelectron transport chain. In prokaryotes, these proteins are found in the cell's inner membrane.[42] These proteins use the energy released from passing electrons fromreduced molecules like NADH onto oxygen to pump protons across a membrane.[43]
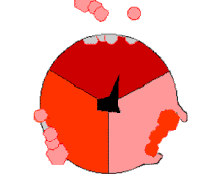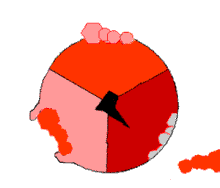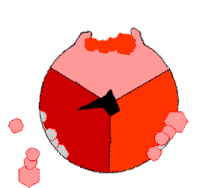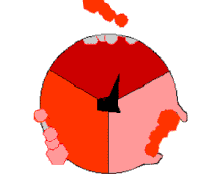
Mechanism of ATP synthase. ATP is shown in red, ADP and phosphate in pink and the rotating stalk subunit in black.
Pumping protons out of the mitochondria creates a proton concentration differenceacross the membrane and generates anelectrochemical gradient.[44] This force drives protons back into the mitochondrion through the base of an enzyme called ATP synthase. The flow of protons makes the stalk subunit rotate, causing the active site of the synthase domain to change shape and phosphorylateadenosine diphosphate – turning it into ATP.[17]
Energy from inorganic compounds
Chemolithotrophy is a type of metabolism found in prokaryotes where energy is obtained from the oxidation of inorganic compounds. These organisms can use hydrogen,[45]reduced sulfur compounds (such as sulfide,hydrogen sulfide and thiosulfate),[1] ferrous iron (FeII)[46] or ammonia[47] as sources of reducing power and they gain energy from the oxidation of these compounds with electron acceptors such as oxygen or nitrite.[48] These microbial processes are important in globalbiogeochemical cycles such as acetogenesis,nitrification and denitrification and are critical for soil fertility.[49][50]
Energy from light
The energy in sunlight is captured by plants,cyanobacteria, purple bacteria, green sulfur bacteria and some protists. This process is often coupled to the conversion of carbon dioxide into organic compounds, as part of photosynthesis, which is discussed below. The energy capture and carbon fixation systems can however operate separately in prokaryotes, as purple bacteria and green sulfur bacteria can use sunlight as a source of energy, while switching between carbon fixation and the fermentation of organic compounds.[51][52]
In many organisms the capture of solar energy is similar in principle to oxidative phosphorylation, as it involves the storage of energy as a proton concentration gradient. This proton motive force then drives ATP synthesis.[17] The electrons needed to drive this electron transport chain come from light-gathering proteins called photosynthetic reaction centres or rhodopsins. Reaction centers are classed into two types depending on the type of photosynthetic pigmentpresent, with most photosynthetic bacteria only having one type, while plants and cyanobacteria have two.[53]
In plants, algae, and cyanobacteria,photosystem II uses light energy to remove electrons from water, releasing oxygen as a waste product. The electrons then flow to thecytochrome b6f complex, which uses their energy to pump protons across the thylakoidmembrane in the chloroplast.[30] These protons move back through the membrane as they drive the ATP synthase, as before. The electrons then flow through photosystem Iand can then either be used to reduce the coenzyme NADP+, for use in the Calvin cycle, which is discussed below, or recycled for further ATP generation.[54]
Anabolism
Anabolism is the set of constructive metabolic processes where the energy released by catabolism is used to synthesize complex molecules. In general, the complex molecules that make up cellular structures are constructed step-by-step from small and simple precursors. Anabolism involves three basic stages. First, the production of precursors such as amino acids,monosaccharides, isoprenoids andnucleotides, secondly, their activation into reactive forms using energy from ATP, and thirdly, the assembly of these precursors into complex molecules such as proteins,polysaccharides, lipids and nucleic acids.
Organisms differ according to the number of constructed molecules in their cells.Autotrophs such as plants can construct the complex organic molecules in cells such as polysaccharides and proteins from simple molecules like carbon dioxide and water.Heterotrophs, on the other hand, require a source of more complex substances, such as monosaccharides and amino acids, to produce these complex molecules. Organisms can be further classified by ultimate source of their energy: photoautotrophs and photoheterotrophs obtain energy from light, whereas chemoautotrophs and chemoheterotrophs obtain energy from inorganic oxidation reactions.
Carbon fixation
Photosynthesis is the synthesis of carbohydrates from sunlight and carbon dioxide (CO2). In plants, cyanobacteria and algae, oxygenic photosynthesis splits water, with oxygen produced as a waste product. This process uses the ATP and NADPH produced by the photosynthetic reaction centres, as described above, to convert CO2into glycerate 3-phosphate, which can then be converted into glucose. This carbon-fixation reaction is carried out by the enzyme RuBisCOas part of the Calvin – Benson cycle.[55] Three types of photosynthesis occur in plants, C3 carbon fixation, C4 carbon fixation and CAM photosynthesis. These differ by the route that carbon dioxide takes to the Calvin cycle, with C3 plants fixing CO2 directly, while C4 and CAM photosynthesis incorporate the CO2 into other compounds first, as adaptations to deal with intense sunlight and dry conditions.[56]
In photosynthetic prokaryotes the mechanisms of carbon fixation are more diverse. Here, carbon dioxide can be fixed by the Calvin – Benson cycle, a reversed citric acid cycle,[57] or the carboxylation of acetyl-CoA.[58][59] Prokaryotic chemoautotrophs also fix CO2 through the Calvin – Benson cycle, but use energy from inorganic compounds to drive the reaction.[60]
Carbohydrates and glycans
In carbohydrate anabolism, simple organic acids can be converted into monosaccharidessuch as glucose and then used to assemblepolysaccharides such as starch. The generation of glucose from compounds likepyruvate, lactate, glycerol, glycerate 3-phosphate and amino acids is calledgluconeogenesis. Gluconeogenesis converts pyruvate to glucose-6-phosphate through a series of intermediates, many of which are shared with glycolysis.[37] However, this pathway is not simply glycolysis run in reverse, as several steps are catalyzed by non-glycolytic enzymes. This is important as it allows the formation and breakdown of glucose to be regulated separately, and prevents both pathways from running simultaneously in a futile cycle.[61][62]
Although fat is a common way of storing energy, in vertebrates such as humans thefatty acids in these stores cannot be converted to glucose throughgluconeogenesis as these organisms cannot convert acetyl-CoA into pyruvate; plants do, but animals do not, have the necessary enzymatic machinery.[63] As a result, after long-term starvation, vertebrates need to produce ketone bodies from fatty acids to replace glucose in tissues such as the brain that cannot metabolize fatty acids.[64] In other organisms such as plants and bacteria, this metabolic problem is solved using theglyoxylate cycle, which bypasses thedecarboxylation step in the citric acid cycle and allows the transformation of acetyl-CoA to oxaloacetate, where it can be used for the production of glucose.[63][65]
Polysaccharides and glycans are made by the sequential addition of monosaccharides byglycosyltransferase from a reactive sugar-phosphate donor such as uridine diphosphate glucose (UDP-glucose) to an acceptorhydroxyl group on the growing polysaccharide. As any of the hydroxyl groups on the ring of the substrate can be acceptors, the polysaccharides produced can have straight or branched structures.[66] The polysaccharides produced can have structural or metabolic functions themselves, or be transferred to lipids and proteins by enzymes called oligosaccharyltransferases.[67][68]
Fatty acids, isoprenoids and steroids
Simplified version of the steroid synthesis pathway with the intermediates isopentenyl pyrophosphate (IPP),dimethylallyl pyrophosphate (DMAPP), geranyl pyrophosphate (GPP) and squalene shown. Some intermediates are omitted for clarity.
Fatty acids are made by fatty acid synthasesthat polymerize and then reduce acetyl-CoA units. The acyl chains in the fatty acids are extended by a cycle of reactions that add the acyl group, reduce it to an alcohol, dehydrateit to an alkene group and then reduce it again to an alkane group. The enzymes of fatty acid biosynthesis are divided into two groups: in animals and fungi, all these fatty acid synthase reactions are carried out by a single multifunctional type I protein,[69] while in plantplastids and bacteria separate type II enzymes perform each step in the pathway.[70][71]
Terpenes and isoprenoids are a large class of lipids that include the carotenoids and form the largest class of plant natural products.[72]These compounds are made by the assembly and modification of isoprene units donated from the reactive precursors isopentenyl pyrophosphate and dimethylallyl pyrophosphate.[73] These precursors can be made in different ways. In animals and archaea, the mevalonate pathway produces these compounds from acetyl-CoA,[74] while in plants and bacteria the non-mevalonate pathway uses pyruvate and glyceraldehyde 3-phosphate as substrates.[73][75] One important reaction that uses these activated isoprene donors is steroid biosynthesis. Here, the isoprene units are joined together to makesqualene and then folded up and formed into a set of rings to make lanosterol.[76]Lanosterol can then be converted into other steroids such as cholesterol andergosterol.[76][77]
Proteins
Organisms vary in their ability to synthesize the 20 common amino acids. Most bacteria and plants can synthesize all twenty, but mammals can only synthesize eleven nonessential amino acids, so nine essential amino acids must be obtained from food.[7]Some simple parasites, such as the bacteriaMycoplasma pneumoniae, lack all amino acid synthesis and take their amino acids directly from their hosts.[78] All amino acids are synthesized from intermediates in glycolysis, the citric acid cycle, or the pentose phosphate pathway. Nitrogen is provided by glutamateand glutamine. Amino acid synthesis depends on the formation of the appropriate alpha-keto acid, which is then transaminated to form an amino acid.[79]
Amino acids are made into proteins by being joined together in a chain of peptide bonds. Each different protein has a unique sequence of amino acid residues: this is its primary structure. Just as the letters of the alphabet can be combined to form an almost endless variety of words, amino acids can be linked in varying sequences to form a huge variety of proteins. Proteins are made from amino acids that have been activated by attachment to atransfer RNA molecule through an ester bond. This aminoacyl-tRNA precursor is produced in an ATP-dependent reaction carried out by anaminoacyl tRNA synthetase.[80] This aminoacyl-tRNA is then a substrate for theribosome, which joins the amino acid onto the elongating protein chain, using the sequence information in a messenger RNA.[81]
Nucleotide synthesis and salvage
Nucleotides are made from amino acids, carbon dioxide and formic acid in pathways that require large amounts of metabolic energy.[82] Consequently, most organisms have efficient systems to salvage preformed nucleotides.[82][83] Purines are synthesized asnucleosides (bases attached to ribose).[84]Both adenine and guanine are made from the precursor nucleoside inosinemonophosphate, which is synthesized using atoms from the amino acids glycine,glutamine, and aspartic acid, as well asformate transferred from the coenzymetetrahydrofolate. Pyrimidines, on the other hand, are synthesized from the base orotate, which is formed from glutamine and aspartate.[85]
Comments
Post a Comment